MIT MAESTRO Study
Mucosal And systEmic Signatures Triggered by Responses to infectious Organisms (MAESTRO)
Leveraging leading edge technology through collaborations with academic and industry partners to define novel biomarkers of Lyme disease and Long COVID and advance understanding of chronic illness following infection.
What are we doing?
For our MIT MAESTRO Study, we are recruiting participants with:
- Acute Lyme Disease
- Chronic Symptoms After Acute Lyme Disease
- Long COVID
- Healthy Volunteers
Planning for 240 participants in 4 groups with 60 participants in each group. Specifically, we are looking at immune responses to infection in different areas of the body like the blood and saliva. We also want to see if we can identify genetic material of pathogens in various body fluids (saliva, urine, and blood, plus optional throat swab, vaginal/menstrual samples, and sweat).
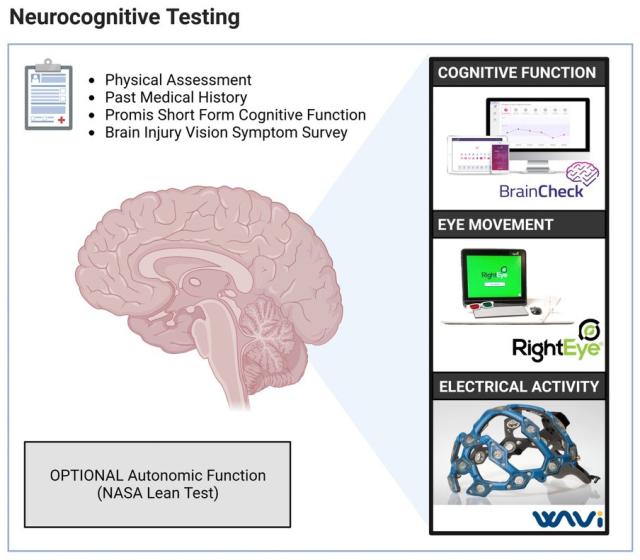
We will be testing eye movement, in addition to cognitive function testing and a hypermobility assessment, as the bacteria that causes Lyme disease (Borrelia burgdorferi) and the virus that causes COVID-19 (SARS-CoV-2) can both invade the brain and nerves. Patients also frequently report debilitating neurological symptoms.
Given the current weaknesses in diagnostic testing for Lyme disease, we are also curious to see if RightEye is able to detect subtle abnormalities in eye movement early in acute Lyme disease to potentially identify infection.
Specific inclusion/exclusion criteria will be screened for in the eligibility survey. We updated this criteria because it was important to us to not exclude probable cases.
Are you eligible?
First, fill out our survey today to see if you are eligible for our study. If you meet the study inclusion criteria for one of the groups we are researching, we will contact you by email to let you know you’ve been selected to participate!
* We have received a large amount of interest in the study. If you are interested in participating, please send us an email and we will add you to a waitlist to complete the eligibility survey. Thank you!
Virtual Visit: E-Consent
If you meet inclusion criteria and are eligible to participate in our study, we will contact you via email to schedule a Zoom virtual visit. In the email will be a copy of the consent form.
At this visit, you will meet with a clinical research professional to thoroughly go over the consent process, the specifics of the study, risks/benefits of participation, and answer any questions you have.
The e-consent virtual visit usually takes about 45 minutes.
Survey Says!
If you have consented to participate in the study, we will then send you some surveys to complete regarding additional demographics, past medical history, tick exposure, COVID exposure and a review of systems/current symptoms.
The surveys take approximately 30–60 minutes to complete.
Before you come to MIT for your research visit, we will also have you complete a short baseline cognitive assessment on your computer at home (BrainCheck). This should take about 10 minutes.
In-Person Visit at MIT
At the in-person MIT Visit you will arrive at the MIT Center for Clinical and Translational Research and the study team will greet you.
You will have your vital signs taken, complete the eye movement testing (RightEye) and brain activity test (WAVi). You will be offered an optional skin integrity testing (Nevisense) and autonomic function testing (NASA lean test).
You will then have samples collected. All participants will self collect a saliva sample and a urine sample. Our clinical research professionals will then take a blood sample. We are also collecting OPTIONAL additional samples of throat swabs, sweat (Macroduct Advanced), and/or a self vaginal/menstrual collection.
The in-person MIT visit usually takes about 3.5-4 hours.